 The FDA describes the goal of the current Good Manufacturing Practice (cGMP), Hazard Analysis, and Risk-Based Preventive Controls for Human Food Rule (the “PC Rule”) as being to establish a food safety system in which industry systematically implements measures that are known to be effective in preventing contamination (FDA Voice, Jan. 7, 2016). To this end, the rule mandates that facilities conduct a hazard analysis to identify the “known or reasonably foreseeable” hazards associated with the foods that they produce. A complete and accurate hazard analysis is the crucial first step in designing an effective food safety plan.
The FDA describes the goal of the current Good Manufacturing Practice (cGMP), Hazard Analysis, and Risk-Based Preventive Controls for Human Food Rule (the “PC Rule”) as being to establish a food safety system in which industry systematically implements measures that are known to be effective in preventing contamination (FDA Voice, Jan. 7, 2016). To this end, the rule mandates that facilities conduct a hazard analysis to identify the “known or reasonably foreseeable” hazards associated with the foods that they produce. A complete and accurate hazard analysis is the crucial first step in designing an effective food safety plan.
In the PC Rule, the FDA says that the information needed to carry out the food safety hazard analysis can be found through “experience, illness data, scientific reports, and other information.” While illness data are unquestionably valuable, they only describe a small part of the food safety universe. The CDC estimates that the affected food is identified for only about 5 percent of all foodborne illnesses. Further, illnesses and injuries caused by physical hazards and by most chemical hazards are not tracked by any public health agency. Illness data, which concern foods at the end of the distribution and preparation chain, cannot be used to identify manufacturing problems except in rare cases (i.e., outbreaks with successful trace back).
Recall information, on the other hand, can often be used to identify food safety problems at the point of production. Recall information provides actionable feedback to food manufactures that can be directly  applied to process evaluation and improvement. While there is some overlap between illness and recall data, the unambiguous relationship between hazards and foods in the recall data makes this information substantially more useful than other data sources.
applied to process evaluation and improvement. While there is some overlap between illness and recall data, the unambiguous relationship between hazards and foods in the recall data makes this information substantially more useful than other data sources.
The most extensive publicly available data on food recalls are the weekly enforcement reports issued by the FDA. Although there is often a long lag between the time a recall is initiated and time it is reported in an enforcement report, the information contained in the 2015 enforcement reports shows how useful these data can be.
The year 2015 was another busy year for FDA food recalls. There were a total of 577 food-related recalls in 2015, which is similar in number in each of the previous two years (Table 1). In FDA speak, each recall is an incident and each incident may involve recalls for multiple products. The number of recalls per month ranged from 23 to 104 (Figure 1). The time between the initiation of a recall and the date that it appeared in an enforcement report ranged from 12 to 917 days, with an average of 68 days. It is not clear why it takes so long for some incidents to be reported in an enforcement report. This lag is different from the lag reported recently by GAO that occurs between the time that FDA becomes aware of a problem and the time that a recall is started.
The level of public health concern, as judged by FDA, for each recall is indicated by the recall class (I, II, or III). Recalls can also be classified according to the type of hazard involved as described in the PC Rule (biological, chemical [which includes allergens], or physical hazards). Table 2 shows both classifications for the 2015 FDA recalls.
 There were 39 recalls caused by physical hazards in 2015. Of these, 35 were Class II; none were considered to be Class I. Metal (14) and plastic (9) fragments accounted for more than half of these recalls.
There were 39 recalls caused by physical hazards in 2015. Of these, 35 were Class II; none were considered to be Class I. Metal (14) and plastic (9) fragments accounted for more than half of these recalls.
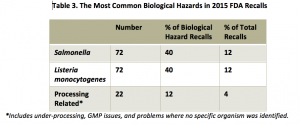
Among the 180 recalls caused by biological hazards, most were caused by either Salmonella or Listeria monocytogenes, 40 percent each (Table 3). Processing problems such as under-processing, spoilage, or GMP-related issues that were not linked to specific pathogens were responsible for 12 percent of the recalls involving biological hazards. Seventy-one percent of the recalls caused by biological hazards were Class I.
There were 357 recalls caused by chemical hazards. Although chemical hazards include a wide range of problems, three-quarters of these recalls were caused by undeclared allergens (Table 4). In 2015, undeclared allergens caused 46 percent of all FDA food recalls, which is similar to past years when approximately half of all food recalls were linked to food allergens. Approximately 60 percent of allergen recalls were Class I and about 10 percent were Class III. This year, a number of recalls early in the year were caused by finding peanut protein in imported cumin. About half of the FDA cumin/peanut recalls were Class I, one was Class III, and the rest were Class II. Although the root cause of the problem was never discovered, or at least never made public, these recalls serve as a reminder of the potential impact of economic adulteration on high value imported products such as spices.
The year 2015 was also busy for the USDA Food Safety and Inspection Service (FSIS). In 2015 there were 150 FSIS recalls, which is a substantial increase from 94 the
year before. As with FDA, a substantial proportion of these recalls were Class II and more than half the recalls were caused by chemical hazards. Undeclared allergens caused 58 recalls, 39 percent of the total for the year. Approximately 20 percent of the FSIS recalls were caused by food that was “produced without the benefit of inspection” or “imported without the benefit of inspection,” which emphasizes the different regulatory authorities for FDA and USDA. Like FDA, there were multiple recalls linked to the peanut in cumin problem early in the year. Unlike FDA, all but one of these recalls were Class I.
The Canadian Food Inspection Agency (CFIA) makes information available on foods recalled in Canada. Despite the different government and regulatory structure, the overall recall pattern in 2015 was very similar to that seen in the U.S. (Table 6). Also, like in the U.S., about half the recalls were caused by undeclared allergens (47 percent in Canada), including several related to cumin.
The fact that on average for the three agencies, about 50 percent of recalls were Class II points out how important recall information is for PC and HACCP assessments. In the U.S., information from sources such as the CDC Foodborne Outbreak Online Database and the FDA Reportable Food Registry Annual Report is often considered to be an accurate and complete description of foodborne public health risks. However, both of these sources only include information on situations that did or would lead to Class I recalls. This means that any problem that would give rise to a Class II recall, or that involves a hazard not tracked by the public health reporting system, is not represented in either data set although there may be a public health risk. In addition, the publically available data in both of these resources is historical rather than “real time.” In contrast, FDA, FSIS, and CFIA all make recall data available on a daily or weekly basis. This means that recall data are more up-to-date and can be used effectively to assess emerging or rapidly changing situations.
Many companies monitor recall announcements as a current awareness tool should one of their suppliers, customers, or competitors be involved in a recall. However, it is clear that a deeper look at recall information provides important insights that can be used to help identify “known or reasonably foreseeable” hazards and to determine which of these require preventive controls.
Dr. Gendel is vice president of Division of Food Allergens for IEH Laboratories and Consulting Group. Reach him at [email protected].
(Editor’s Note: all charts/figures provided courtesy of Dr. Gendel)




Leave a Reply